Our research focuses
- on twin-arginine translocation (Tat) systems that transport folded proteins across prokaryotic cytoplasmic membranes,
- on the periplasmic maturation and modification of pyoverdines in fluorescent pseudomonads,
- on membrane stress sensing and response mediated by the phage shock protein (Psp) system in Escherichia coli,
- and on phage holin regulation and mechanism.
Tat System
Tat transport – Transport of folded proteins across the cytoplasmic membrane
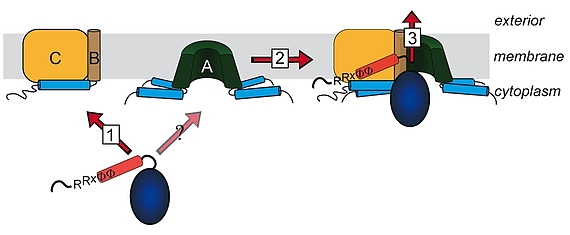
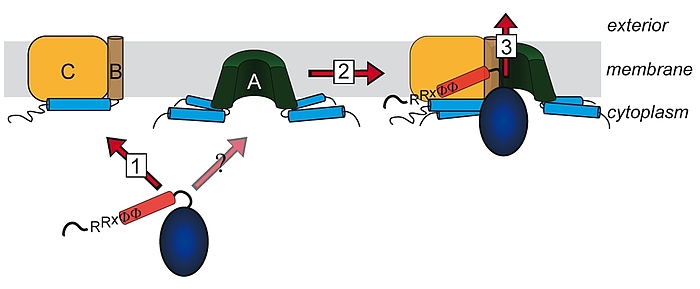
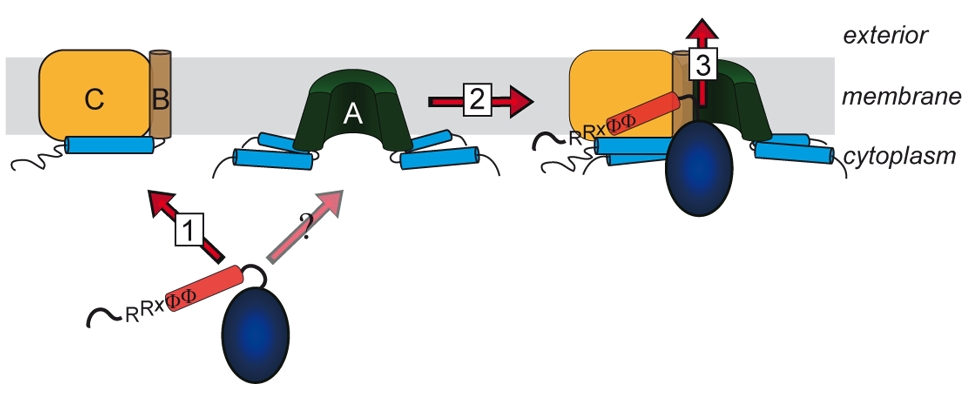
For many years it was believed that only unfolded protein chains can be translocated across membranes. This view changed during the 90ies when it became apparent that some proteins are translocated as oligomers, with a transport-initializing signal peptide at only one subunit. The co-translocation of protein subunits that do not possess their own signal peptide clearly indicated that the oligomer must have been formed prior to translocation. The components of the corresponding translocation system that transports folded proteins across the cytoplasmic membrane have been identified and named TatA, TatB, and TatC. TatA and TatB are quite similar, and there exist also “minimal” Tat systems that are made of TatA and TatC. TatAB-family proteins are unusual in structura, as they possess a very short membrane anchor, followed by an amphipathic helix on the cytoplasmic side and only a few more residues with relevance for Tat transport. TatC has six trans-membrane helices and binds tightly to TatB, and less tightly to TatA. We work on Tat since the 90ies, have proposed the membrane-weakening and pulling mechanism for Tat transport in 2003, and biochemically analyze the Tat mechanism in all aspects.
PSP System
The Psp membrane stress response
Bacteria often experience environmental changes that harm the integrity of the membranes and therefore can be regarded as "membrane stress". In Escherichia coli, it has been observed for many of these conditions that a regulon is upregulated that consists of the pspABCDE operon and the monocistronic pspG gene. The corresponding promoters depend on the alternative sigma factor sigma 54, which is activated by the bacterial enhancer binding protein PspF that is divergently encoded upstream of the pspABCDE operon. PspF in turn is regulated by the first component encoded by the pspABCDE operon, PspA. PspA is the key regulator that somehow integrates the various stress signals to result in the desired activity of PspF and consequently in the desired response on sigma 54 dependent psp transcription level. It is believed that the PspB and PspC components sense membrane stress signals and interact with PspA to transmit these signals. We are interested in the signal sensing by the various Psp components, the signal transmission to PspA, and the mode of the signal integration by PspA. To analyze the aspects, we combine a wide range of structural and molecular biological tools.
Pyoverdine
Periplasmic pyoverdine maturation and modification
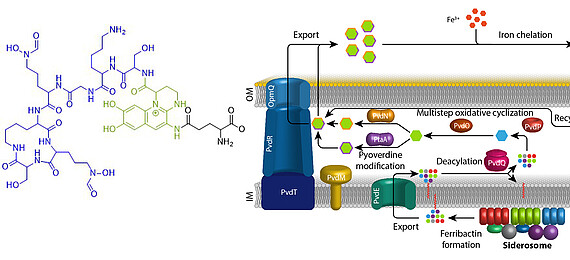
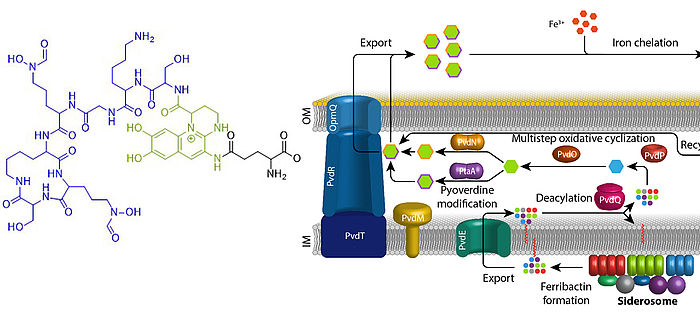
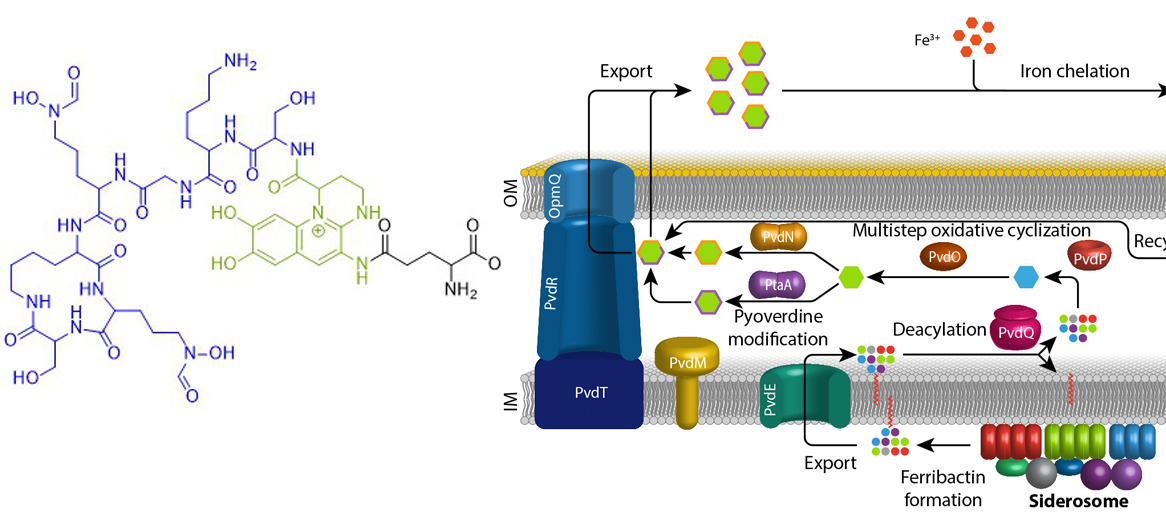
Iron is an essential element for almost all living organisms on earth. Due to little solubility of ferric iron in most oxic environments, specific mechanisms have evolved that ensure iron supply. Fluorescent pseudomonads that live in host evironments, such as human pathogenic bacteria or plant endophytes, face extreme iron limitation. Under these conditions, they produce and secrete pyoverdines. Pyoverdines are fluorescent high-affinity siderophores that chelate ferric iron and then are taken up again to deliver the bound iron to the bacteria. Pyoverdines are generated from cytoplasmically synthesized non-ribosomal peptides, so-called ferribactins, that are converted to fluorescent and modified pyoverdines inside the periplasm. We study the periplasmic maturation and modification of pyoverdines. So far we identified the physiological roles of three out of five involved enzymes, PvdO, PtaA, and PvdN. We also could specify more precisely the role of a fourth enzyme, PvdP, which forms the fluorophore together with PvdO. Our aim is to clarify the complete biochemistry of periplasmic pyoverdine maturation and modification.
Holine
Phage holins, their regulation and mechanism
Phage infection and host lysis shape the population and diversity of bacteria in many habitats. Host lysis has been investigated for decades and the players are well-known. However, structural insight is still scarce, and mechanistic aspects for host lysis regulation and the lysis mechanism per se are still far from being understood. Coming from membrane protein complex analysis, we started several years ago to work on the regulation and mechanism of holins, which are pore-forming large associations of membrane proteins. Holin pores permit the release of endolysins into the periplasm, where they prompt cell lysis by hydrolyzing the peptidoglycan cell wall. Holins can do this either directly - by generating pores that permit the release of the endolysins from the cytoplasm into the periplasm - or they do this indirectly by abolishing the membrane potential, which is triggering the release of endolysins that had been already transported by the Sec translocon and membrane anchored by a so-called "signal anchor release domain" (SAR domain). The first class of (large) pore-forming holins are the "canonical" holins, whereas the second class of (small) pore-forming holins are so-called "pinholins". We currently work on canonical holins.
Contact


30419 Hannover




